Firefly Core-3562JQ is a system-on-module based on Rockchip RK3562J SoC – the industrial-grade version of the Rockchip RK3562 quad-core Cortex-A53 Tablet SoC – designed to operate in the -40 to 85°C industrial temperature range and suitable for digital signage, industrial control systems, industrial PLCs, energy data concentrators, smart healthcare, self-service terminals, and more. The CPU module features up to 8GB RAM, 64GB flash, and a Rockchip RK809-5A PMIC, plus three 80-pin board-to-board connectors exposing video output and input interfaces, audio (SPDIF, PDM, I2S) interfaces, networking (GbE and Fast Ethernet), various USB interface, a 1-lane PCIe 2.1 interface, and more. Firefly Core-3562JQ specifications: SoC – Rockchip RK3562J CPU – Quad-core Arm Cortex-A53 processor @ up to 1.2 GHz (against 2.0 GHz for the Rockchip RK3562) GPU – Arm Mali-G52 EE with support for OpenGL ES 3.2, Vulkan 1.1, OpenCL 2.0 AI accelerator – None listed in the specs (Rockchip RK3562 comes […]
Quectel FGH100M Wi-Fi HaLow module based on Morse Micro MM6108 receives CE and FCC Certifications
In a collaboration, Quectel Wireless Solutions and Morse Micro have developed the FGH100M a Wi-Fi HaLow module, powered by Morse Micro’s MM6108 SoC. This module has achieved CE certification in Europe and FCC approval in the US, meeting high safety and environmental standards. After reading through the press release, I initially thought the 802.11.ah WiFi, known as WiFi HaLow, would be similar to other LPWAN standards like LoRaWAN or Sigfox. However, further research showed that Wi-Fi HaLow, operating in the 900 MHz band, was first announced in 2014 and got its name in 2016. However, in the following years, there wasn’t much interest in this wireless standard. But starting from 2021, it’s becoming more popular, and we’ve seen many HaLow-based products like a mini PCIe card, a WiFi HaLow development board, and a gateway kit to extend the range of IP cameras, and also ALFA Network’s AHPI7292S Raspberry Pi HAT […]
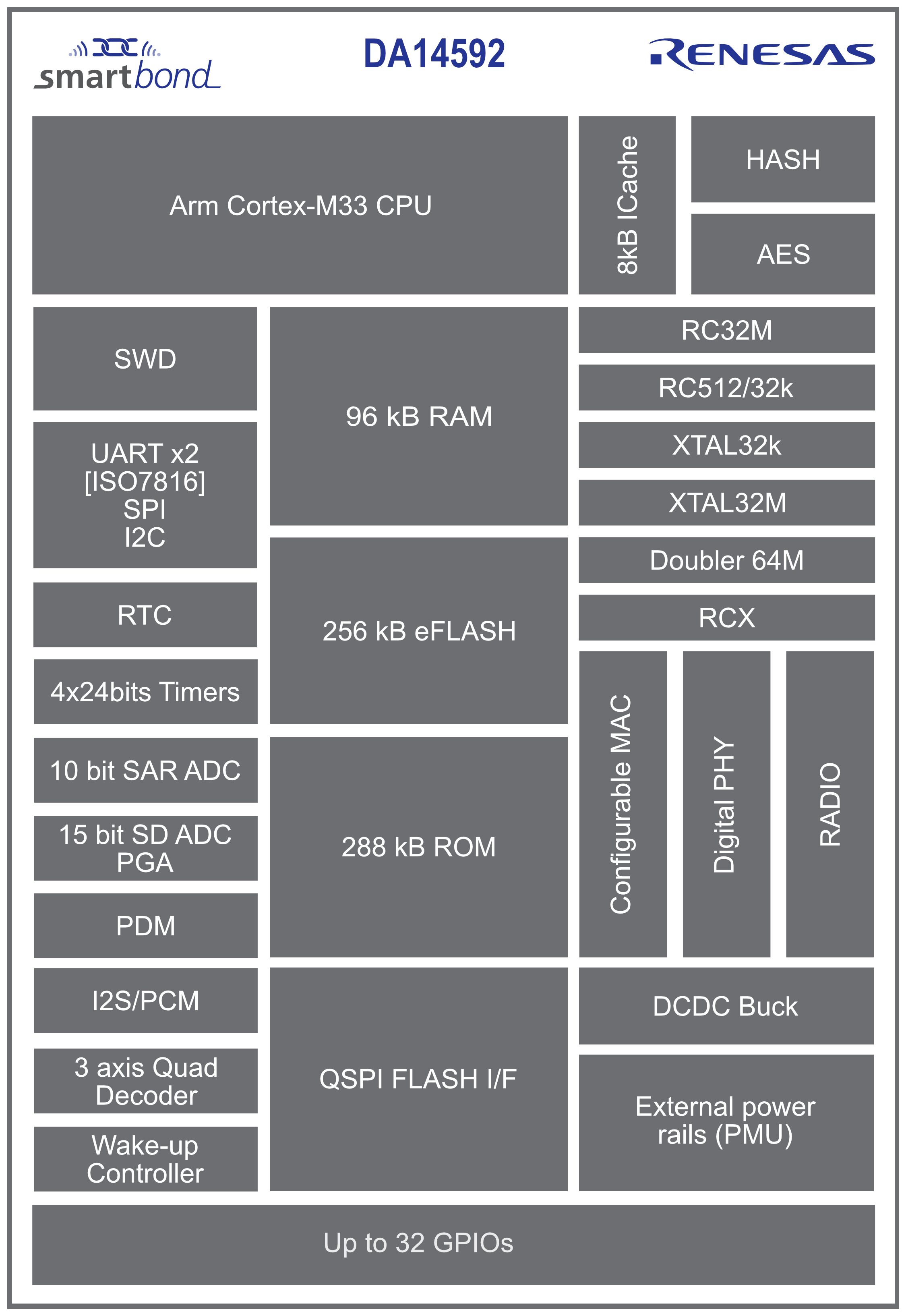
Renesas releases the DA14592, a new dual-core Cortex-M33/M0+ BLE chip with integrated flash
The DA14592 System-on-Chip (Soc) is Renesas’ “lowest power consumption and smallest, multi-core Bluetooth LE device.” According to the company, this chip’s design was achieved by making careful tradeoffs between on-chip memory and die size. The chip offers an ultra-low power mode that uses only 2.3mA when transmitting radio signals at 0dBm and consumes 1.2mA to receive radio signals. It also supports a hibernation mode that uses about 90nA and can extend the operating life of battery-powered products. The active mode is best for processing-intensive products and uses 34µA for every MHz of CPU clock. Projected key applications for the DA14592 chip include crowd-sourced location (CSL) tracking as used in Apple’s Airtags, remote control units, human interface devices, asset tracking, IoT end nodes, data logging, connected health, and activity tracking. Renesas DA14592 specifications: CPU cores: Cortex-M33F running at up to 64MHz as application core Cortex-M0+ at 64MHz as configurable MAC Memory: […]
u-blox MAYA-W3 industrial wireless module supports WiFi 6/6E and Bluetooth 5.4 with LE Audio
We’ve also covered two IoT-focused WiFi 6 and Bluetooth LE chips in the last week with Synaptics SYN43711 chipset and Gigadevice GD32VW553 RISC-V WiSoC, but there’s more and u-blox has recently introduced the MAYA-W3 WiFi 6/6E and Bluetooth 5.4 module with LE Audio support and designed for industrial applications. The MAYA-W3 module offers some flexibility as it’s designed on the upcoming Infineon AIROC CYW5551x WiFi 6E and Bluetooth 5.4 chip family supporting 2.4 GHz only (CYW55511), 2.4 GHz and 5 GHz (CYW55512), and a 2.4 GHz, 5 GHz, and 6 GHz with the tri-band CYW55512 chipset. u-blox also provides three antenna options for each model with two U.FL connectors, two antenna pin connectors, or an embedded PCB antenna. u-Blox MAYA-W3 specifications: Chipset – Infineon AIROC CYW5551x CYW55511 for 2.4 GHz WiFi 6 CYW55512 for 2.4 and 5 GHz WiFi 6 CYW55513 for 2.4, 5, and 6 GHz WiFi 6E Wireless […]
Android 14 released, source code hits AOSP
Google has just released Android 14 for supported devices such as Google Pixel phones and pushed the source code to AOSP (the Android Open-Source Project). Most of the changes to the fourteenth version of the Android operating system were introduced with the first Android 14 developer preview – released in February 2023 – which included performance improvements, better privacy and security, and additional user-side customization options. Some of the new features unveiled since the first Android 14 developer preview include: AI-generated wallpapers using text-to-image diffusion models to help users easily create unique wallpapers HDR images with Ultra HDR (Android 13 already supported HDR videos) Built-in Health Connect support to let people track their fitness, health, and wellness levels across apps in a secure way respecting privacy. Android 14 encourages users to set a six-digit PIN (or longer) to improve security. Improved accessibility with vision-and hearing-inclusive features such as an enhanced […]
Realtek RTL8852BE based WiFi 6 and Bluetooth 5.2 module targets Smart Home, industrial and healthcare applications
Telit Cinterion WE310K6-P is a dual-band Wi-Fi 6 and Bluetooth 5.2 based on the Realtek RTL8852BE wireless controller and designed to be integrated into connected devices for Smart Home, medical, industrial, and smart city applications. The WE310K6-P wireless is available in either LGA or M.2 module form factors and can operate in the -40°C to +85°C temperature range which should make it suitable for a wide range of Linux devices. Telit Cinterion WE310K6-P specifications: Wireless chipset – Realtek RTL8852BE Wi-Fi WiFi 6 802.11 a/b/g/n/ac/ax (2.4GHz, 5GHz) with 20MHz, 40MHz, or 80MHz bandwidth Dual-stream spatial multiplexing up to 1201 Mbps data rate Tx output – 19 dBm @ 2.4 GHz, 18 dBm @ 5 GHz Wi-Fi 802.11 e, h, k, i support Security features such as WPA3 and integrated crypto hardware Bluetooth Version 5.2 Dual mode with Bluetooth 2.1 Classic Bluetooth Low Energy up to 2 Mbps Bluetooth LE Audio (CIS) […]
NEWRACOM NRC7394 WiFi HaLow SoC delivers higher power efficiency and cost-effectiveness
NEWRACOM has just introduced the NRC7394 Wi-Fi HaLow Arm Cortex-M3 SoC with higher power efficiency and lower cost than the previous generation NRC7292 Cortex-M3/M0 HaLow SoC and available in a 6x6mm package. I first wrote about the 802.11ah standard in 2014. Also known as the WiFi HaLow (consumers name), it operates in the 900 MHz band, offers a longer range and lower power consumption for items like IP cameras, and the first products came to market in 2021. I was expecting a flood of new WiFi HaLow devices in 2022 in my year 2021 round-up and it was not exactly a prescient prediction as it never happened. But maybe the new NRC7394 SoC will help make WiFi HaLow devices more popular by lowering the costs and further improving battery life. NEWRACOM NRC7394 key features: CPU – Arm Cortex-M3 core @ 32 MHz for IEEE 802.11ah WLAN and application Connectivity Full […]
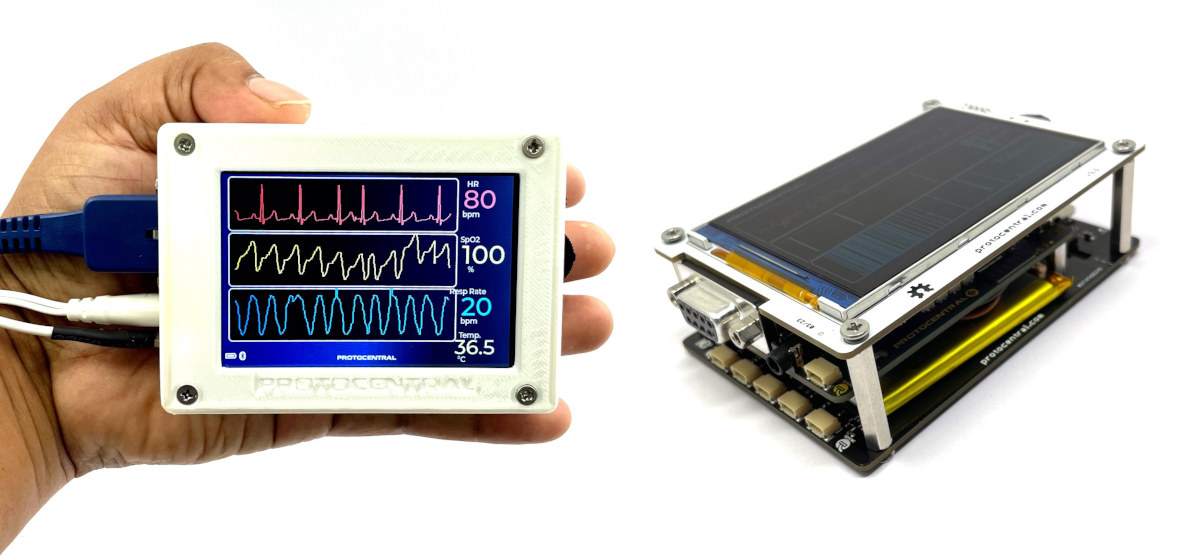
HealthyPi 5 WiFi & BLE biosignal-acquisition sensor platform captures body temperature, ECG, PPG, SpO₂, and other vitals (Crowdfunding)
HealthyPi 5 is an open-source sensor platform for biosignal acquisition based on Raspberry Pi RP2040 microcontroller and ESP32-C3 WiFi & BLE module used to capture vitals such as electrocardiogram (ECG), respiration, photoplethysmography (PPG), oxygen saturation (SpO₂), and body-temperature data. It is a complete redesign of the HealthyPi v4 Raspberry Pi HAT with many of the same features. While the HealthyPi 5 also follows the Raspberry Pi HAT form factor and can be connected to a Raspberry Pi SBC to analyze the data, it can also be used as a standalone device with the processing handled by the RP2040 dual-core Cortex-M0+ microcontroller and connectivity through an ESP32-C3 wireless module, and data visualized on a 3.5-inch SPI display or a smartphone over WiFi or Bluetooth. HealthyPi 5 specifications: MCU – Raspberry Pi RP2040 dual-core Arm Cortex-M0+ microcontroller @ 133 MHz with 264 KB SRAM Wireless Module – ESP32-C3 RISC-V module with 2.4 […]